Footprinting protein–DNA complexes using the hydroxyl radical
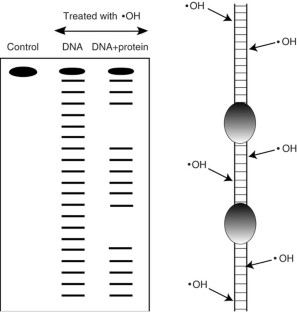
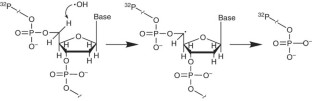
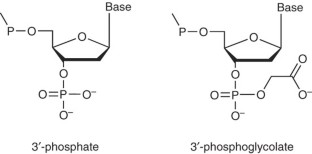
Hydroxyl radical footprinting has been widely used for studying the structure of DNA and DNA–protein complexes. The high reactivity and lack of base specificity of the hydroxyl radical makes it an excellent probe for high-resolution footprinting of DNA–protein complexes; this technique can provide structural detail that is not achievable using DNase I footprinting. Hydroxyl radical footprinting experiments can be carried out using readily available and inexpensive reagents and lab equipment. This method involves using the hydroxyl radical to cleave a nucleic acid molecule that is bound to a protein, followed by separating the cleavage products on a denaturing electrophoresis gel to identify the protein-binding sites on the nucleic acid molecule. We describe a protocol for hydroxyl radical footprinting of DNA–protein complexes, along with a troubleshooting guide, that allows researchers to obtain efficient cleavage of DNA in the presence and absence of proteins. This protocol can be completed in 2 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Analysis of protein-DNA interactions in chromatin by UV induced cross-linking and mass spectrometry
Article Open access 16 October 2020
Electro-elution-based purification of covalent DNA–protein cross-links
Article 18 June 2024
Deciphering molecular interactions by proximity labeling
Article 11 January 2021
References
- Tullius, T.D. Physical studies of protein-DNA complexes by footprinting. Annu. Rev. Biophys. Biophys. Chem.18, 213–237 (1989). ArticleCASPubMedGoogle Scholar
- Hampshire, A.J., Rusling, D.A., Broughton-Head, V.J. & Fox, K.R. Footprinting: a method for determining the sequence selectivity, affinity and kinetics of DNA-binding ligands. Methods42, 128–140 (2007). ArticleCASPubMedGoogle Scholar
- Adilakshmi, T., Lease, R.A. & Woodson, S.A. Hydroxyl radical footprinting in vivo: mapping macromolecular structures with synchrotron radiation. Nucleic Acids Res.34, e64 (2006). ArticlePubMedPubMed CentralGoogle Scholar
- Urbach, A.R. & Waring, M.J. Visualising DNA: footprinting and 1-2D gels. Mol. BioSyst.1, 287–293 (2005). ArticleCASPubMedGoogle Scholar
- Galas, D.J. & Schmitz, A. DNase footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res.5, 3157–3170 (1978). ArticleCASPubMedPubMed CentralGoogle Scholar
- Brenowitz, M., Senear, D.F., Shea, M.A. & Ackers, G.K. Quantitative DNase footprint titration: a method for studying protein-DNA interactions. Meth. Enzymol.130, 132–181 (1986). ArticleCASGoogle Scholar
- Drew, H.R. & Travers, A.A. DNA structural variations in the E. coli tyrT promoter. Cell37, 491–502 (1984). ArticleCASPubMedGoogle Scholar
- Sawadogo, M. & Roeder, R.G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell43, 165–175 (1985). ArticleCASPubMedGoogle Scholar
- Tullius, T.D., Dombroski, B.A., Churchill, M.E. & Kam, L. Hydroxyl radical footprinting: a high-resolution method for mapping protein-DNA contacts. Meth. Enzymol.155, 537–558 (1987). ArticleCASGoogle Scholar
- Tullius, T.D. & Dombroski, B.A. Hydroxyl radical 'footprinting': high-resolution information about DNA-protein contacts and application to Lambda repressor and Cro protein. Proc. Natl. Acad. Sci. USA83, 5469–5473 (1986). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tullius, T.D. DNA footprinting with hydroxyl radical. Nature332, 663–664 (1988). ArticleCASPubMedGoogle Scholar
- Pogozelski, W.K. & Tullius, T.D. Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. Chem. Rev.98, 1089–1108 (1998). ArticleCASPubMedGoogle Scholar
- Price, M.A. & Tullius, T.D. Using hydroxyl radical to probe DNA structure. Meth. Enzymol.212, 194–219 (1992). ArticleCASGoogle Scholar
- Price, M.A. & Tullius, T.D. How the structure of an adenine tract depends on sequence context: a new model for the structure of TnAn DNA sequences. Biochemistry32, 127–136 (1993). ArticleCASPubMedGoogle Scholar
- Ganunis, R.M., Guo, H. & Tullius, T.D. Effect of the crystallizing agent 2-methyl-2,4-pentanediol on the structure of adenine tract DNA in solution. Biochemistry35, 13729–13732 (1996). ArticleCASPubMedGoogle Scholar
- Churchill, M.E., Tullius, T.D., Kallenbach, N.R. & Seeman, N.C. Holliday recombination intermediate is twofold symmetric. Proc. Natl. Acad. Sci. USA85, 4653–4656 (1988). ArticleCASPubMedPubMed CentralGoogle Scholar
- Tullius, T.D. & Greenbaum, J.A. Mapping nucleic acid structure by hydroxyl radical cleavage. Curr. Opin. Chem. Biol.9, 127–134 (2005). ArticleCASPubMedGoogle Scholar
- Churchill, M.E., Hayes, J.J. & Tullius, T.D. Detection of drug binding sites by hydroxyl radical footprinting. Relationship of distamycin binding to nucleosome positions on the 5S RNA gene of Xenopus. Biochemistry29, 6043–6050 (1990). ArticleCASPubMedGoogle Scholar
- Mah, S.C., Townsend, C.A. & Tullius, T.D. Hydroxyl radical footprinting of calicheamicin. Relationship of DNA binding to cleavage. Biochemistry33, 614–621 (1994). ArticleCASPubMedGoogle Scholar
- Tullius, T.D. & Dombroski, B.A. Iron(II) EDTA used to measure the helical twist along any DNA molecule. Science230, 679–681 (1985). ArticleCASPubMedGoogle Scholar
- Udenfriend, S., Clark, C.T., Axelrod, J. & Brodie, B.B. Ascorbic acid in aromatic hydroxylation. I. A model system for aromatic hydroxylation. J. Biol. Chem.208, 731–740 (1954). CASPubMedGoogle Scholar
- Fenton, H.J.H. Oxidation of tartaric acid in the presence of iron. J. Chem. Soc.65, 899–910 (1894). ArticleCASGoogle Scholar
- Shcherbakova, I., Mitra, S., Beer, R.H. & Brenowitz, M. Fast Fenton footprinting: a laboratory-based method for the time-resolved analysis of DNA, RNA and proteins. Nucleic Acids Res.34, e48 (2006). ArticlePubMedPubMed CentralGoogle Scholar
- Shcherbakova, I. & Brenowitz, M. Monitoring structural changes in nucleic acids with single residue spatial and millisecond time resolution by quantitative hydroxyl radical footprinting. Nat. Protoc.3, 288–302 (2008). ArticleCASPubMedGoogle Scholar
- Hayes, J.J., Kam, L. & Tullius, T.D. Footprinting protein-DNA complexes with gamma-rays. Meth. Enzymol.186, 545–549 (1990). ArticleCASGoogle Scholar
- Ottinger, L.M. & Tullius, T.D. High-resolution in vivo footprinting of a protein-DNA complex using γ-radiation. J. Am. Chem. Soc.122, 5901–5902 (2000). ArticleCASGoogle Scholar
- Greenbaum, J.A., Pang, B. & Tullius, T.D. Construction of a genome-scale structural map at single-nucleotide resolution. Genome Res.17, 947–953 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Greenbaum, J.A., Parker, S.C. & Tullius, T.D. Detection of DNA structural motifs in functional genomic elements. Genome Res.17, 940–946 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Steitz, T.A. Structural studies of protein-nucleic acid interactions: the sources of sequence specific binding. Q. Rev. Biophys.23, 205–280 (1990). ArticleCASPubMedGoogle Scholar
- Pabo, C.O. & Sauer, R.T. Transcription factors: structural families and principles of DNA recognition. Annu. Rev. Biochem.61, 1053–1095 (1992). ArticleCASPubMedGoogle Scholar
- Carey, J. Gel retardation. Meth. Enzymol.208, 103–117 (1991). ArticleCASGoogle Scholar
- Lane, D., Prentki, P. & Chandler, M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol. Rev.56, 509–528 (1992). CASPubMedPubMed CentralGoogle Scholar
- Fried, M.G. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis10, 366–376 (1989). ArticleCASPubMedGoogle Scholar
- Garner, M.M. & Revzin, A. The use of gel electrophoresis to detect and study nucleic acid-protein interactions. Trends Biochem. Sci.11, 395–396 (1986). ArticleCASGoogle Scholar
- Fried, M.G. & Crothers, D.M. Equilibria and kinetics of Lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res.9, 6505–6525 (1981). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hellman, L.M. & Fried, M.G. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat. Protoc.2, 1849–1861 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Woodbury, C.P. Jr. & von Hippel, P.H. On the determination of deoxyribonucleic acid-protein interaction parameters using the nitrocellulose filter-binding assay. Biochemistry22, 4730–4737 (1983). ArticleCASPubMedGoogle Scholar
- Nirenberg, M. & Leder, P. RNA codewords of protein synthesis. Science145, 1399–1407 (1964). ArticleCASPubMedGoogle Scholar
- Jones, O.W. & Berg, P. Studies on the binding of RNA polymerase to polynucleotides. J. Mol. Biol.22, 199–209 (1966). ArticleCASPubMedGoogle Scholar
- Yarus, M. & Berg, P. Recognition of tRNA by aminoacyl tRNA synthetases. J. Mol. Biol.28, 479–490 (1967). ArticleCASPubMedGoogle Scholar
- Vrana, K.E., Churchill, M.E., Tullius, T.D. & Brown, D.D. Mapping functional regions of transcription factor TFIIIA. Mol. Cell. Biol.8, 1684–1696 (1988). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hayes, J.J., Tullius, T.D. & Wolffe, A.P. The structure of DNA in a nucleosome. Proc. Natl. Acad. Sci. USA87, 7405–7409 (1990). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dixon, W.J., Inouye, C., Karin, M. & Tullius, T.D. CUP2 binds in a bipartite manner to upstream activation sequence c in the promoter of the yeast copper metallothionein gene. J. Biol. Inorg. Chem.1, 451–459 (1996). ArticleCASGoogle Scholar
- Jamison McDaniels, C.P., Jensen, L.T., Srinivasan, C., Winge, D.R. & Tullius, T.D. The yeast transcription factor Mac1 binds to DNA in a modular fashion. J. Biol. Chem.274, 26962–26967 (1999). ArticleCASPubMedGoogle Scholar
- Bashkin, J.S., Hayes, J.J., Tullius, T.D. & Wolffe, A.P. Structure of DNA in a nucleosome core at high salt concentration and at high temperature. Biochemistry32, 1895–1898 (1993). ArticleCASPubMedGoogle Scholar
- Widlund, H.R. et al. Nucleosome structural features and intrinsic properties of the TATAAACGCC repeat sequence. J. Biol. Chem.274, 31847–31852 (1999). ArticleCASPubMedGoogle Scholar
- Draganescu, A., Levin, J.R. & Tullius, T.D. Homeodomain proteins: what governs their ability to recognize specific DNA sequences? J. Mol. Biol.250, 595–608 (1995). ArticleCASPubMedGoogle Scholar
- Kimball, A.S., Milman, G. & Tullius, T.D. High-resolution footprints of the DNA-binding domain of Epstein-Barr virus nuclear antigen 1. Mol. Cell. Biol.9, 2738–2742 (1989). ArticleCASPubMedPubMed CentralGoogle Scholar
- Danford, A.J., Wang, D., Wang, Q., Tullius, T.D. & Lippard, S.J. Platinum anticancer drug damage enforces a particular rotational setting of DNA in nucleosomes. Proc. Natl. Acad. Sci. USA102, 12311–12316 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Draganescu, A. & Tullius, T.D. The DNA binding specificity of engrailed homeodomain. J. Mol. Biol.276, 529–536 (1998). ArticleCASPubMedGoogle Scholar
- Buchman, C., Skroch, P., Dixon, W.J., Tullius, T.D. & Karin, M. A single amino acid change in CUP2 alters its mode of DNA binding. Mol. Cell. Biol.10, 4778–4787 (1990). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kimball, A.S., Kimball, M.L., Jayaram, M. & Tullius, T.D. Chemical probe and missing nucleoside analysis of Flp recombinase bound to the recombination target sequence. Nucleic Acids Res.23, 3009–3017 (1995). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dixon, W.J. et al. Hydroxyl radical footprinting. Meth. Enzymol.208, 380–413 (1991). ArticleCASGoogle Scholar
- Lutter, L.C. Precise location of DNase I cutting sites in the nucleosome core determined by high resolution gel electrophoresis. Nucleic Acids Res.6, 41–56 (1979). ArticleCASPubMedPubMed CentralGoogle Scholar
- Maxam, A.M. & Gilbert, W. Sequencing end-labeled DNA with base specific chemical cleavages. Meth. Enzymol.65, 499–560 (1980). ArticleCASGoogle Scholar
- Das, R., Laederach, A., Pearlman, S.M., Herschlag, D. & Altman, R.B. SAFA: semi-automated footprinting analysis software for high-throughput quantification of nucleic acid footprinting experiments. RNA11, 344–354 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Current Protocols in Nucleic Acid Chemistry (ed. Harkins, E.W.) (John Wiley & Sons, Inc., Somerset, New Jersey, 2005).
- Vinson, C.R., Sigler, P.B. & McKnight, S.L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science246, 911–916 (1989). ArticleCASPubMedGoogle Scholar
- Dixon, W.J. Determination of the protein-DNA interactions of two transcription activators, the yeast Copper Protein 2 and the human Growth Hormone Factor 1. DNA-binding models for the Copper "Fist" and POU domain. (Dissertation) (The Johns Hopkins University, Baltimore, Maryland, 1992). Google Scholar
- Balasubramanian, B., Pogozelski, W.K. & Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA95, 9738–9743 (1998). ArticleCASPubMedPubMed CentralGoogle Scholar
- Shadle, S.E. et al. Quantitative analysis of electrophoresis data: novel curve fitting methodology and its application to the determination of protein-DNA binding constant. Nucleic Acids Res.25, 850–860 (1997). ArticleCASPubMedPubMed CentralGoogle Scholar
Acknowledgements
We thank Dr. Wendy Dixon for producing the CUP2 footprint shown in Figure 2.








